

Looking for a sign to switch?
In a long-term safety study, results showed people could safely switch from other preventative treatments without risking more attacks.

In the open-label study, the majority of people switched safely without an overall increase in HAE attacks
DAWNZERA was evaluated in people who switched from lanadelumab, berotralstat, or a C1-INH.*
HAE mean attack reduction was seen regardless of prior preventative treatment.†
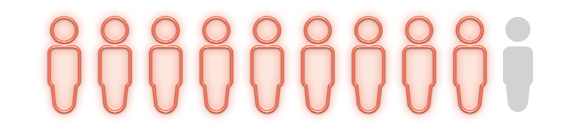
 people in the study who used the Angioedema Control Test (AECT) reported being well-controlled after switching to DAWNZERA (secondary endpoint interim analysis at Week 16)
people in the study who used the Angioedema Control Test (AECT) reported being well-controlled after switching to DAWNZERA (secondary endpoint interim analysis at Week 16)
of people surveyed preferred DAWNZERA over their previous preventative treatment, citing better disease control, less time required for treatment, and reduced injection pain as reasons for their preference.§
Consider the DAWNZERA difference

confusion on switching
Stopping your current treatment and starting DAWNZERA was evaluated and outlines a path forward.
mixing or syringes
DAWNZERA offers the convenience of a self-administered autoinjector. No mixing or syringes, and although refrigeration is recommended, it can be stored at room temperature for 6 weeks.‖
frequent medicine reminders
Dosing once every 4 weeks or every 8 weeks.
If you are new to DAWNZERA and want to see if it’s right for you, you may be eligible to receive a one-time, one-dose supply of DAWNZERA at no cost. Talk to your doctor about the Free Trial Program available through Ionis Every Step™.¶
Learn moreThe Ionis Every Step™ Free Trial Program (“FTP”), sponsored by Ionis Pharmaceuticals, Inc., the manufacturer of DAWNZERA™ (donidalorsen), provides a 4-week supply of DAWNZERA™ to patients who meet FTP eligibility requirements and who agree to the FTP terms and conditions by submitting a signed consent form. FTP is a free trial offer, intended solely to allow patients who are new to DAWNZERA™ to determine with their healthcare provider whether DAWNZERA™ is right for them. There is no obligation to continue use of DAWNZERA™ after the free trial has been completed. To be eligible, a patient must: (1) reside in the United States or Puerto Rico; (2) have a valid prescription for DAWNZERA™ for an FDA-approved indication; and (3) be a new patient who has not previously been treated with DAWNZERA™. This offer is limited to one use per patient per lifetime and is non-transferable. DAWNZERA™ supplied through the FTP will (i) be dispensed only by a pharmacy designated by Ionis; (ii) be delivered only to the patient’s home address (no P.O. boxes) and (iii) not exceed a 4-week supply; no refills are available through the FTP. It is unlawful for any person to sell, purchase, trade, barter, or export DAWNZERA™ supplied through the FTP or make an offer to do so. Patients, pharmacists, and prescribers may not seek reimbursement, either directly or indirectly, for DAWNZERA™ supplied through the FTP from health insurance or any third-party payer, including Medicare, Medicaid, and commercial insurance plans. Patients must not count the value of the FTP product as an expense incurred for purposes of determining patient out-of-pocket costs under any health insurance program, including Medicare Part D True Out-of-Pocket (“TrOOP”) costs.
THE FTP IS NOT HEALTH INSURANCE. The FTP is not a discount, rebate, coupon, cost-sharing program, or other form of financial assistance. Obtaining DAWNZERA™ through the FTP is not contingent on any past, present, or future purchase of DAWNZERA™. To continue a patient on therapy after such one-time use, a separate prescription must be written by the healthcare provider. Use of the FTP is void where prohibited by law and where use is prohibited by the patient’s insurance provider. Ionis reserves the right to rescind, revoke, or amend the FTP at any time without notice.
C1-INH=C1 esterase inhibitor.
‖DAWNZERA autoinjectors can be stored in their original cartons at room temperature (up to 86 °F [30 °C]) for up to 6 weeks. Do not expose your DAWNZERA autoinjector to heat, do not freeze it, and protect it from direct light.
Hear from people who made the switch
Switching HAE treatments is a big decision, but you're not alone. Hear first-hand experiences from those who made the decision to switch.
Ready to switch to DAWNZERA?
Download the Doctor Discussion Guide to help start the conversation and to see if DAWNZERA may be right for you.
Download guide
From helping you start to keeping you on track
lonis Every Step connects you with a dedicated Patient Education Manager# right from the start to help guide you through your treatment journey—one step at a time.
#Patient Education Managers cannot give medical advice and do not replace your medical team. Contact your doctor for any medical concerns.
